
Discussion with UKTIS is recommended.Department of Pharmacy Practice, Narayana Pharmacy College, Nellore, Andhra Pradesh, India

Where exposure to OPs has occurred in pregnancy, enhanced maternal and fetal monitoring may be warranted. Where a specific antidote is clinically indicated this should not be withheld on account of pregnancy. Management of the pregnant patient should be the same as for the non-pregnant patient. Maternal toxicity following OP exposure is likely to be a major determinant of risk to the fetus. There are no guidelines regarding the treatment of OP poisoning during pregnancy. Where maternal toxicity is present, particularly when exposure occurs close to term or delivery, there may be a risk of neonatal toxicity. Due to the lack of data and the possible adverse associations reported with chronic, low dose exposure to pesticides in general, an increase in risk cannot be ruled out. Numerous studies have raised concerns about possible adverse effects on pregnancy outcome following chronic low dose OP pesticide exposure due to residential or occupational proximity to agricultural areas.ĭata on acute OP exposure/poisoning in pregnancy are limited to case reports.
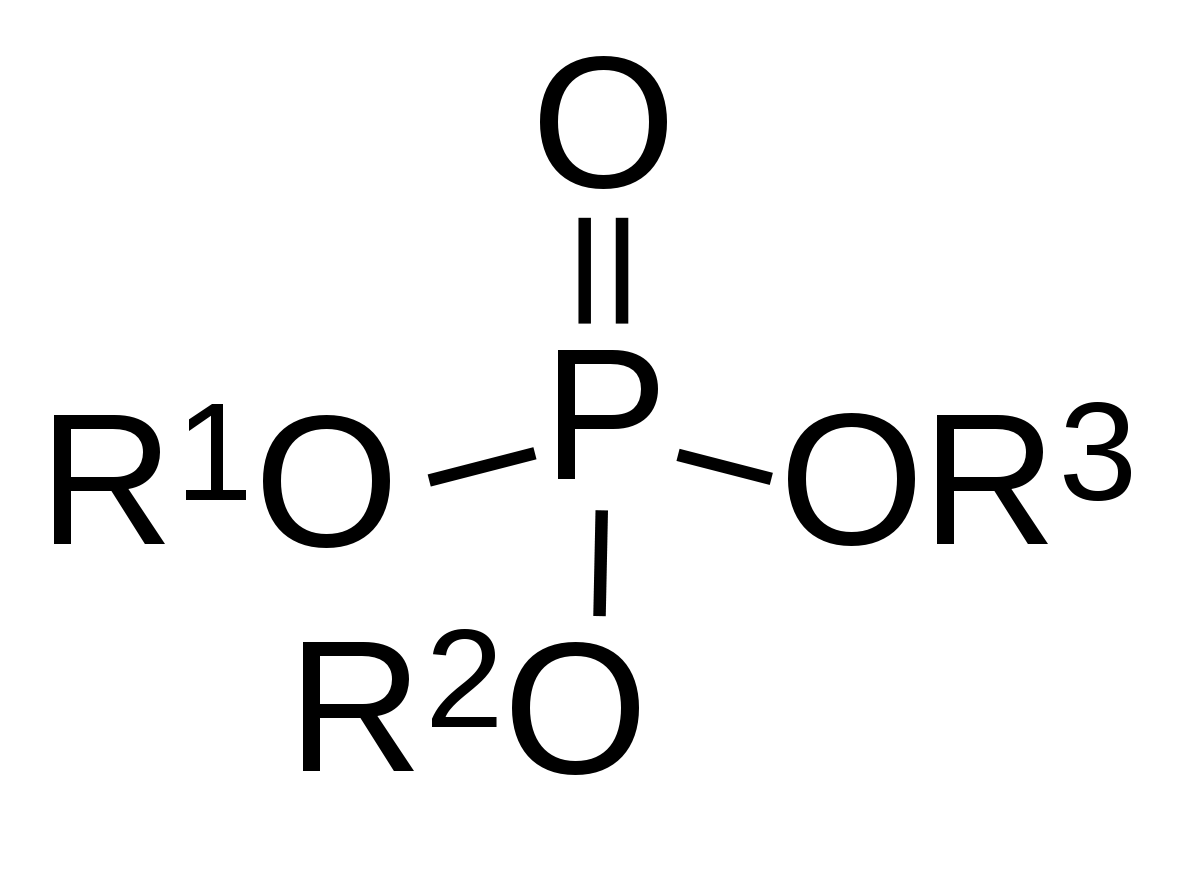
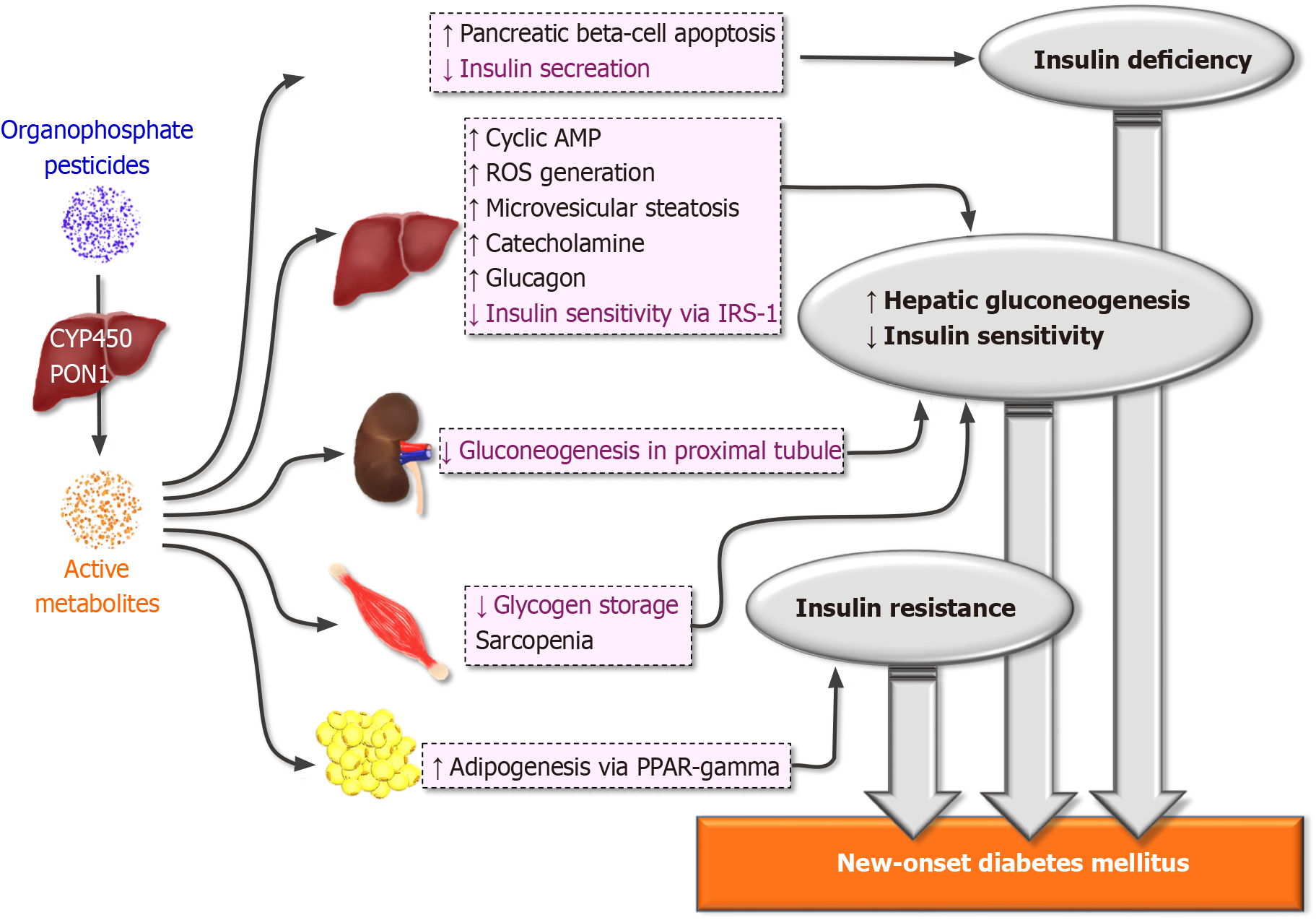
OPs are often diluted in an organic solvent and exposure can occur from ingestion, inhalation, dermal, and ocular exposure. OPs are also used in chemical warfare as nerve agents. Organophosphates (OPs) are esters of phosphoric acid widely used as domestic and industrial insecticides and pesticides.


 0 kommentar(er)
0 kommentar(er)
